Nickel has been discovered in almost 2,000-year-old metallic artifacts.
Axel Cronstedt, a Swedish scientist, was the first to identify and isolate it as an element in 1751. It rose to prominence in the 19th century in plating and alloys like "nickel silver" (German silver), in which it is alloyed with copper and zinc. This alloy was called for its color rather than its silver content.
Miners in Germany discovered in the 15th century a brown-red ore that they thought contained copper. Because they could not collect copper from it, they named it Kupfernickel or Devils' Copper.
Nickel alloyed with copper was first used in coins in the United States in 1857. Although the "nickel" was not made of pure nickel, pure nickel was used for coins in Switzerland in 1881.
Early in the 20th century, stainless steel was developed, and nickel was discovered to play a highly advantageous role in many of the typical grades, which continues to this day. Nickel-based alloys were discovered to have great corrosion resistance and the ability to tolerate high temperatures, making them ideal for chemical plants and allowing the practical implementation of the jet engine. Nickel's demand has risen dramatically over the last century as a result of these advancements. This is still the case today, because of the critical function nickel plays in a variety of applications.
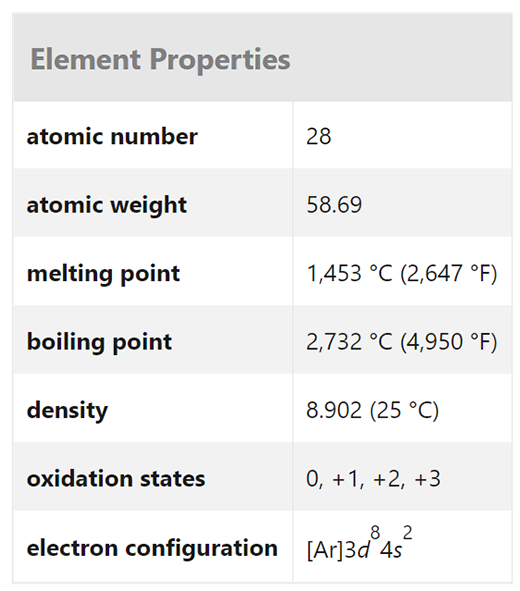
Nickel (Ni) is a chemical element that is a ferromagnetic metal belonging to Group 10 (VIIIb) of the periodic table and is highly resistant to oxidation and corrosion.
Nickel is a silvery-white, lustrous metallic element that occurs naturally. It is the fifth most common element on the planet, and it is abundant in the crust and core. Nickel, like iron, is a common element in meteorites and is also found in trace amounts in plants, animals, and oceans.
While the earth's crust contains 80 parts per million of nickel, the earth's core is primarily made up of a nickel-iron alloy.
Properties and Occurrence
Nickel has exceptional physical and chemical qualities, making it a necessary component in tens of thousands of products. Its main application is alloying, especially with chromium and other metals to create stainless and heat-resistant steels.
Nickel is a white, hard-silver metal that forms cubic crystals. It is malleable and ductile, and it is strong and corrosion-resistant. The metal is a good heat and electrical conductor, and it has magnetic characteristics below 345°C.
Nickel is chemically inert in its metallic form. It is unaffected by concentrated nitric acid and alkalis and is insoluble in cold and hot water as well as ammonia. However, it is soluble in weak nitric acid and only sparingly in dilute hydrochloric and sulfuric acids.
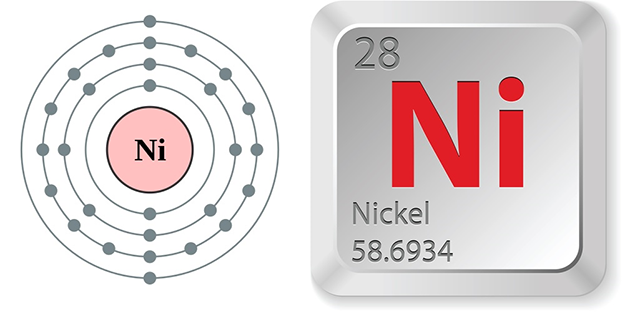
Electron configuration and elemental properties of nickel

Nickel is a silvery-white metal that is robust and harder than iron. It is well-known for its usage in coins, but it is more essential for its various domestic and industrial applications as a pure metal or in alloys. In terrestrial and meteoric deposits, element nickel is found in very small amounts alongside iron.
Nickel is twice as abundant as copper, accounting for around 0.007% of the Earth's crust; it is a common constituent of igneous rocks, however, only a few deposits meet the criteria for commercial interest in terms of concentration, size, and accessibility. Significant quantities are thought to exist in the Earth's central regions. Pentlandite, found with nickel-bearing pyrrhotite, of which certain variants contain 3 to 5% nickel, and chalcopyrite, as well as nickel-bearing laterites, such as garnierite, a magnesium–nickel silicate of variable composition, are the most important sources.
Nickel metallurgy is intricate in its details, many of which change greatly depending on the type of ore being treated. In general, the ore is converted to dinickel trisulfide, Ni2S3 (with nickel in the +3 oxidation state), which is then roasted in air to produce nickel oxide, NiO (+2 state), which is then reduced with carbon to produce the metal. The carbonyl method is used to produce some high-purity nickel.
Nickel (atomic number 28) has the strength and toughness of iron (atomic number 26), but it has the oxidation and corrosion resistance of copper (atomic number 29), which accounts for many of its applications. Nickel is a metal with excellent electrical and thermal conductivity. More than half of the nickel produced is utilized in iron alloys (especially stainless steels), while the balance is used in corrosion-resistant copper alloys and heat-resistant chromium alloys. Nickel can also be found in electrically resistive, magnetic, and a variety of other alloys, including nickel silver (with copper and zinc but no silver). The unalloyed metal is used to cover other metals with protective coatings, particularly by electroplating.
Nickel may be easily manufactured using normal hot and cold working techniques. Nickel reacts slowly with fluorine, gradually creating a protective fluoride layer, and is thus utilized in equipment for handling fluorine gas and corrosive fluorides as a pure metal or in alloys such as Monel. Nickel is ferromagnetic at room temperature, but not as much as iron. It is also less electropositive than iron but dissolves readily in dilute mineral acids.
Nickel-58 (68.27 %), nickel-60 (26.10 %), nickel-61 (1.13 %), nickel-62 (3.59 %), and nickel-64 (0.91 %) are the five stable isotopes found in natural nickel. Its cubic crystal structure is face-centered. Up to 358 °C (676 °F), nickel is ferromagnetic. Because of its remarkable resistance to alkali action, the metal is commonly employed to make containers for concentrated sodium hydroxide solutions. Under normal conditions, nickel reacts slowly with strong acids to liberate hydrogen and create Ni2+ ions.
Compounds
Nickel has oxidation states of 1, 0, +1, +2, +3, and +4 in its compounds, albeit the +2 state is by far the most prevalent. Ni2+ forms a wide range of complexes, including octahedral, trigonal bipyramidal, tetrahedral, and square structures, as well as coordination numbers 4, 5, and 6.
Nickel compounds in the +2 state offer a wide range of industrial applications. Nickel chloride (NiCl2), nickel nitrate (Ni (NO3)2·6H2O), and nickel sulfamate (Ni (SO3NH2)2∙4H2O) are all commonly used in nickel electroplating baths. Nickel sulfate, NiSO4, is also used to make catalysts, ground-coat enamels, and mordants (fixatives) for dyeing and textile printing. For usage in fuel cells and storage batteries, nickel oxide (NiO) and nickel peroxide (Ni2O3) are created. Magnetic cores made of nickel ferrites are used in a variety of electrical equipment, including antennas and transformers.
Nickel sulfide, NiS; nickel arsenide, NiAs; nickel antimonide, NiSb; nickel diarsenide, NiAs2; nickel thioarsenide, NiAsS; and nickel thioantimonide, NiSbS are some of the most common nickel compounds found in nature, where it occurs primarily as minerals in combination with arsenic, antimony, and sulfur. Nickel is in the +2-oxidation state in the sulfide, whereas it is in the +3 state in all of the other compounds mentioned.
Nickel carbonyl, or tetracarbonylnickel, Ni (CO)4, is another major commercial chemical. This zero-oxidation state nickel compound is principally used as a carbon monoxide carrier in the synthesis of acrylates (compounds used in the making of polymers) from acetylene and alcohols. It was the first of a new class of chemicals known as metal carbonyls (1890). Carbon monoxide reacts with finely divided nickel to produce a colorless, volatile liquid with an electrical structure in which the nickel atom is surrounded by 36 electrons. This arrangement is quite similar to that of noble-gas atoms.
Nickel is the 22nd most plentiful element on the planet and the 7th most common transition metal. It is a silvery-white crystalline metal that can be found in meteorites or in ores when mixed with other elements.
Ores can be divided into two categories:
- Laterites: oxide or silicate ores, such as garnierite;
- Sulfides: ores containing around 1.5 % nickel, nickel associated with copper, cobalt, and other metals, such as pentlandite.
Ludwig Mond invented a method for extracting and purifying nickel in 1899. The conversion of nickel oxides to pure nickel metal is known as the "Mond Process." Nickel ores are processed into oxide by a number of steps that include concentration, roasting, and smelting.
Nickel oxide is reacted with water gas, a mixture of H2 and CO, at atmospheric pressure and 50 °C in the first step of the process. As a result, the oxide is converted to impure nickel.
The process can be summarized as follows:
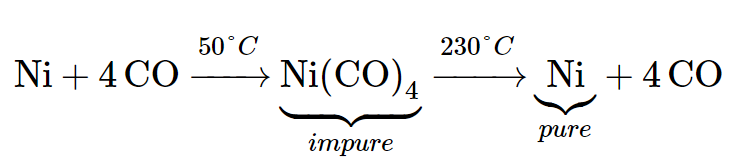
The harmful and volatile chemical nickel tetracarbonyl, Ni (CO)4, is formed when this impure substance reacts with leftover carbon monoxide. When heated to around 230°C, this combination decomposes into pure nickel metal and CO, which can then be recycled. Temperatures and pressures employed in this process might vary a lot from one processing plant to the next. The essential method, as stated, is, nonetheless, universal.
Laterite deposits, which form as a result of intense weathering of surface nickel-rich rocks, and magmatic sulfide deposits are the two most common forms of nickel deposits. Nickel is also found in manganese nodules and crusts on the deep-sea floor, although these are not mined at the moment. Limonite, garnierite, and pentlandite are the most common nickel-bearing minerals.
Norway was the first large-scale nickel smelting site in 1848. Pyrrhotite is a type of nickel ore that was used there. Large nickel resources were discovered in Canada's Sudbury Basin in 1883. This enormous nickel deposit is thought to have formed as a result of an ancient meteor impact. In the early twentieth century, more nickel was discovered in Russia and South Africa, allowing nickel to gain even more traction in the industry.
Exclusive Metal
Only a few products are created entirely of nickel. Instead, nickel is used in industry materials to provide support and stability; it is frequently mixed with other metals to create stronger, shinier, and more durable products. Nickel is widely used to protect weaker metals against corrosion.
Nickel is the metal of choice for super-alloys, metal combinations that are noted for their strength as well as resistance to heat, corrosion, and oxidation, due to its ability to sustain extremely high temperatures. In fact, stainless steels account for around 65 % of nickel production, while the remaining 20 % is used to produce various steel and non-iron alloys for highly specialized military, aerospace, and industrial applications. Other types of applications, such as batteries and electronics, and coins, account for about 9% of the total.
Magnetism
Nickel is one of only four ferromagnetic metals, meaning it attracts magnets and is magnetic in its own right. Iron, cobalt, and gadolinium are the others. Alnico magnets, which are made up of aluminum (Al), nickel (Ni), and cobalt (Co), are extremely strong permanent magnets that retain their polarity even after being heated to a red glow.
Mu-metal is a soft magnetic alloy made up of about 80% nickel and 20% iron (and a dash of molybdenum). Because Mu-metal has a high permeability, it can protect sensitive electronic equipment from static or low-frequency magnetic fields. When Mu-metal is placed between a magnet and a metal, the regular attraction vanishes.